Approximately one third of all people with FTD are genetic, and research has shown that changes in the brain associated with FTD can occur years before any symptoms (or presymptomatically). We need to be able to recognise brain changes in the presymptomatic stage of genetic FTD as clinical trials of potential treatments might target those who have not yet developed symptoms.
Position emission tomography (PET) imaging is a type of brain imaging which allows us to investigate certain molecules in the brain. Rather than changes in the brain’s structure, PET imaging can tell us about changes in the brain’s function. One type of PET imaging we have explored in FTD is PET imaging of glucose metabolism. Brain cells use glucose as a source of energy to support their function, but in all different types of dementia we see reduced glucose metabolism in different areas of the brain. This suggests brain cells become dysfunctional in dementia, and this dysfunction might contribute to eventual cell loss. PET imaging of glucose metabolism in presymptomatic FTD might help us understand which areas of the brain become dysfunctional first.
In a recent study led by Mica Clarke in collaboration with the FTD centre in Quebec (Robert Laforce and Frédéric St-Onge) we explored glucose metabolism in a specific group of people with MAPT mutations who had yet to develop symptoms – presymptomatic carriers of a P301L MAPT mutation. Six carriers of the mutation and 12 individuals without a genetic mutation underwent [18F]FDG PET scans (to measure glucose metabolism) and magnetic resonance imaging (MRI) scans (to measure brain volume).
We found that compared to individuals without a genetic mutation, carriers of a P301L MAPT mutation showed reduced glucose metabolism and reduced brain volume in an area of the brain called the anterior cingulate cortex. The difference in glucose metabolism in this area were marginally better at discriminating those with a mutation versus without a mutation than the difference in volume in this area. However, combining the two measures gave even better diagnostic ability.
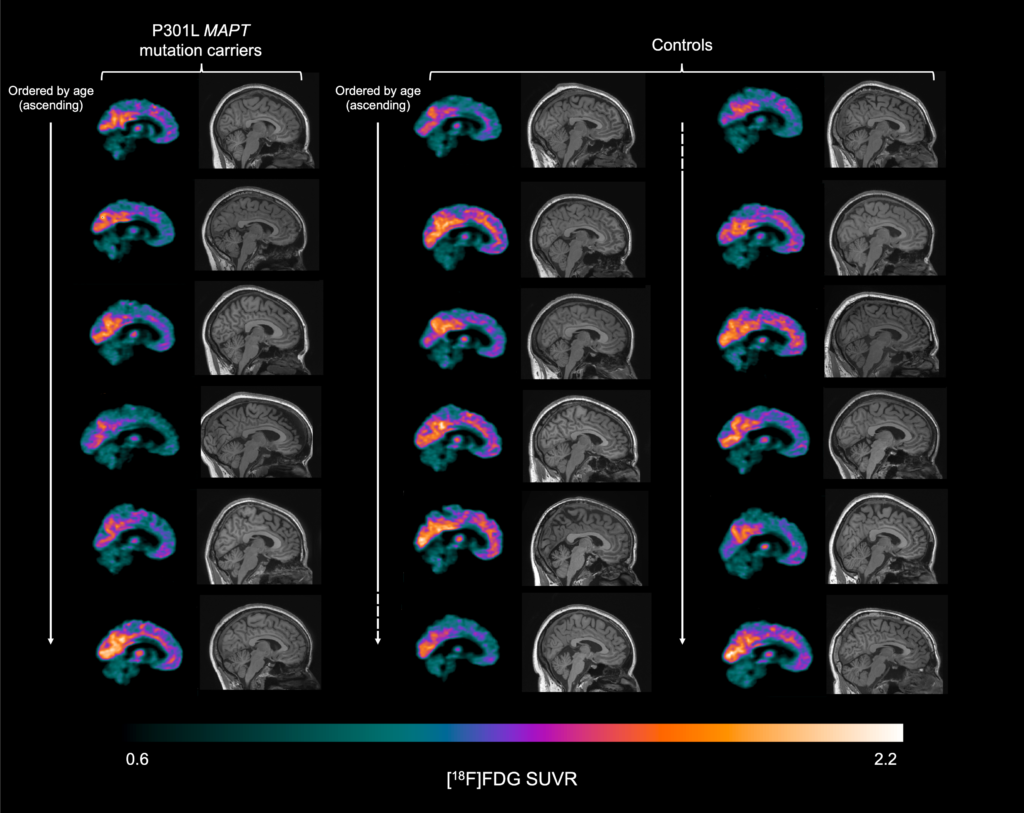
The mutation carriers were estimated to be on average 12.5 years away from symptom onset, suggesting changes in the anterior cingulate cortex are the first to occur in P301L MAPT mutation carriers. This might explain why most P301L MAPT mutation carriers develop behavioural variant FTD (bvFTD), as the anterior cingulate cortex is one of the main brain regions affected in bvFTD. Such early and specific changes in the brain in this group might be helpful in deciding who can enter potential clinical trials and in monitoring how effective those clinical trials are.